pH Levels of Rainwater: When & Why It’s Important
Understanding the pH levels of rainwater is crucial for a variety of environmental and health reasons. pH, which stands for 'potential hydrogen', is a scale used to specify how acidic or basic a water-based solution is. Rainwater is naturally slightly acidic due to the presence of carbon dioxide in the atmosphere, which dissolves into the water and forms carbonic acid. The pH of rainwater is an important indicator of atmospheric purity and can be influenced by various factors including industrial emissions and natural processes.
Over time, the pH of rainwater can be affected by human activities, such as the burning of fossil fuels, which release sulfur dioxide and nitrogen oxides into the atmosphere. These pollutants can lead to the formation of acid rain, which can have harmful effects on plants, aquatic life, and infrastructure. Therefore, monitoring the pH of rainwater is important for protecting ecosystems and man-made structures, as well as for understanding the broader impacts of human activities on the environment.
Key Takeaways
- Rainwater pH indicates atmospheric purity and is influenced by carbon dioxide and human activities.
- Acid rain, with lower pH levels, has negative effects on ecosystems and infrastructure.
- Monitoring rainwater pH is crucial for environmental protection and understanding human impact.
What are “pH Levels”?
pH levels are a critical measure of the acidity or basicity of water.
This is essential for various chemical processes and environmental health. Understanding and being able to manage pH levels is key to utilizing rainwater for various purposes – ensuring quality, effectiveness, and safety.
The pH Scale
The pH scale is a numerical representation running from 0 to 14 that tells you how acidic or basic a solution is. A substance with a pH of 7 is considered neutral, which means it is neither acidic nor basic. Values less than 7 indicate acidic qualities, while values greater than 7 indicate basic (or alkaline) qualities. The scale is logarithmic, so each whole number change means a tenfold increase or decrease in acidity or basicity.
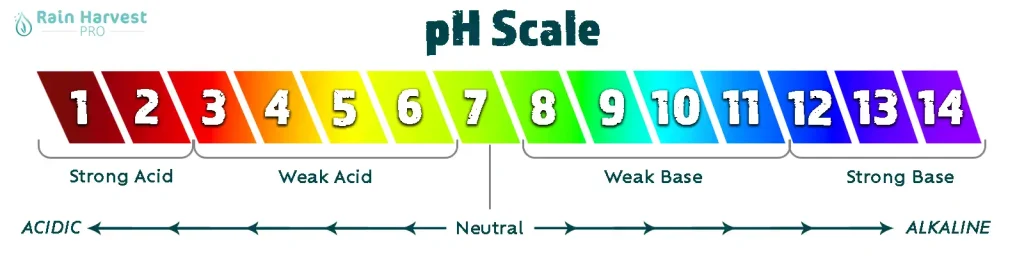
Here are some common substances and their pH values for example:
| Substance | pH Value |
|---|---|
| Battery Acid | 1 |
| Lemon Juice | 2 |
| Vinegar | 3 |
| Black Coffee | 5 |
| Pure Water | 7 |
| Baking Soda Solution | 9 |
| Bleach | 12.5 |
Acidic vs. Basic Solutions
An acidic solution has a pH below 7. The lower the pH value, the more hydrogen ions it contains, and the stronger the acid is. Conversely, a basic solution has a pH above 7, with fewer hydrogen ions and more hydroxide ions than neutral water. Basic solutions are often referred to as alkaline. Acids and bases can neutralize each other; when an acid and base are mixed together in the right proportions, they form a neutral solution with a pH close to 7.
Acidity in Rainwater
Acidity levels in rainwater are critical indicators of both environmental health and the impact of anthropogenic emissions. By understanding the pH of rainwater, you can gain insights into the natural processes affecting our atmosphere and ecosystems as well as how we can impact it as humans.
Measuring the pH of Rainwater
The pH value is used to determine the acidity of rainwater, with 7 being neutral. Acid rain typically has a pH value below 5.6. Reliable measurement of rainwater’s pH can alert you to changes in atmospheric chemistry due to pollutants like sulfur dioxide and nitrogen oxides emanating from fossil fuel combustion. Tools such as pH meters or indicator papers are commonly employed for this task.
Natural and Human-Made Sources
While natural sources such as volcanic gases can contribute to the acidity of rainwater, man-made sources are a significant concern. Emissions from factories, vehicles, and industrial plants release large amounts of sulfur dioxide (SO2) and nitrogen oxides (NOx) into the atmosphere. These compounds can travel long distances, as noted in research discussing the factors controlling the acidity of natural rainwater, before being converted into acids within raindrops.
Effects of Acid Rain on the Environment
When acid rain falls, it can have harmful effects on natural and constructed environments. It leads to the acidification of lakes and streams, which can be devastating to aquatic life. Moreover, it accelerates the deterioration of buildings and cultural monuments, especially those made of limestone and marble, as the chemical characteristics of rainwater have shown. Vegetation can also suffer, as acidic waters strip soil of essential nutrients and release aluminum, leading to stunted growth and reduced productivity.
Chemical Constituents and Reactions
In analyzing the chemistry of rainwater, it is crucial to understand the chemical constituents and reactions that define its pH levels. These are influenced by various substances and processes in the atmosphere.
Role of Carbon Dioxide
When carbon dioxide (CO2) interacts with water (H2O), it leads to the formation of carbonic acid (H2CO3). This weak acid disassociates in the rainwater to form hydrogen ions (H+) and bicarbonate ions (HCO3-), contributing to rainwater’s acidity.
CO2 + H2O ⇌ H2CO3 ⇌ H+ + HCO3-
Nitrogen Oxides and Sulfur Dioxide
The presence of nitrogen oxides (NOx) and sulfur dioxide (SO2) in the atmosphere alters rainwater pH through the creation of strong acids. Nitrogen oxides can transform into nitric acid, while sulfur dioxide may form sulfuric acid upon reaction with water, both increasing rainwater acidity.
2NO2 + H2O → HNO2 + HNO3
SO2 + H2O → H2SO3
Other Relevant Chemical Reactions
All atmospheric substances, including calcium (Ca) and iron (Fe), can influence rainwater composition. Calcium often acts as a neutralizing agent to acidity, while reactions involving iron can produce various complex ions that alter rainwater chemistry. Such processes underscore the importance of understanding the dynamics within the rainwater’s ionic composition.
Rainwater’s Interaction with Materials
Rainwater can markedly influence the materials it comes into contact with, from natural substances like soil and stone to humanmade objects like buildings and plumbing systems.
Effects on Soil and Vegetation
When rainwater trickles through the soil, its pH levels can fundamentally impact soil chemistry. Your soil may become more acidic or alkaline depending on the pH of rainwater, affecting nutrient availability for plants. Acidic rainwater can lead to the leaching of essential nutrients such as calcium and magnesium, which are vital for vegetation. Conversely, in limestone-rich regions, rainwater tends to be more neutralized, which can buffer soil pH and benefit plant growth.
Impact on Buildings and Monuments
Monuments and buildings, particularly those crafted from calcium-based stones such as marble and limestone, are sensitive to the acidic components in rainwater. Acid rain can result in the dissolution of these materials, leading to surface erosion and structural damage over time. In contrast, structures made from more resistant materials like granite are less likely to suffer from rainwater’s effects.
Corrosion in Appliances and Plumbing
Your appliances and plumbing systems, which often consist of metals like copper and components treated with chlorine, can experience corrosion when exposed to acidic rainwater. This is especially true for metal elements that constantly contact with water. Over time, this corrosion can lead to the deterioration of pipes and fixtures, necessitating repairs or replacement to avoid leaks and water damage.
Environmental and Health Implications
Understanding the pH levels of rainwater is crucial because they can have significant impacts on both the environment and human health. Changes in pH can affect aquatic life, terrestrial ecosystems, and the quality of water used for drinking and agricultural purposes.
Consequences for Aquatic Life
Acidic rainwater with a low pH can lead to the acidification of lakes and streams, which can be detrimental to fish and other aquatic organisms. When water becomes too acidic:
- Fish eggs may fail to hatch.
- Aquatic plants struggle to survive due to altered mineral availability.
- Amphibians such as frogs are vulnerable, as their eggs have no protective shell.
Acid Rain and Terrestrial Ecosystems
The impact of acidic rainwater is not limited to aquatic environments; it also endangers terrestrial ecosystems. Specifically:
- Trees become stressed and more susceptible to disease and insect damage.
- Soil chemistry changes, leading to nutrient imbalances and the death of sensitive plants and animals.
- The environment as a whole can suffer from a breakdown in vital ecological interactions and processes.
Implications for Human Health
Your health can indirectly be influenced by the effects of acidic rainwater on water quality. Contaminant yields from metal roofs, for instance, can greatly exceed guideline values for drinking water, as seen in research describing contaminant yields. Moreover:
- Harvesting rainwater for potable use requires careful consideration of quality to minimize health risks.
- Drinking water from sources contaminated with acidic rain can lead to harmful metal exposure.
Testing and Regulation
In evaluating rainwater quality, you’ll find that assessing pH levels holds significant importance. Proper testing and adherence to regulation ensure the safety and suitability of rainwater for various uses.
pH Measurement Techniques
You can measure the pH of rainwater using a range of techniques, each with varying degrees of precision and suitability for different settings. Lab-based tests are highly accurate and can use sophisticated equipment such as pH meters which offer digital readouts for precise measurements. In more educational settings, such as a water science school, simpler methods like litmus paper—a type of water science tool—can provide a quick and less precise acidity assessment. The data gathered from these techniques are crucial for understanding the effect of environmental factors on rainwater acidity levels as monitored by organizations like the National Atmospheric Deposition Program.
- Lab Equipment: Advanced, accurate digital pH meters.
- Litmus Paper: Convenient, color-based pH estimate.
Rainwater pH Testing Standards and Guidelines
When it comes to regulation, the Environmental Protection Agency (EPA) sets critical benchmarks for water quality, including pH levels. While the EPA doesn’t regulate the water quality of residential rainwater harvesting systems, their guidelines serve as a reference point for assessing suitability, typically recommending a pH range of 6.5 to 8.5 for drinking water.
Adherence to such standards also informs the design of rainwater harvesting systems to ensure that the quality of collected water meets the acceptable criteria for usage, whether it’s for irrigation, toilet flushing, or if treated, for drinking.
Remember, consistent testing across different contexts enables a comprehensive understanding of rainwater quality and the adherence to well-established guidelines ensures its safe application.
Water Treatment Methods for Neutralizing Unsafe Water
Understanding the pH levels of rainwater is crucial for addressing the environmental concerns posed by acid rain. Your action can make a significant difference in either directly neutralizing the acidity of rainwater or addressing the root causes to prevent the occurrence of acid rain.
Neutralization Processes
In mitigating acidic rainwater, lime (calcium oxide) and calcium carbonate are often used to adjust the pH level. When you employ these substances to treat acidic bodies of water or soil, they react with the water to form bicarbonate ions, which neutralize the acidity. For example, in a lake, you might add finely ground limestone in what’s known as liming. The calcium compounds react with the acidified water, increasing the pH closer to a neutral level, which is vital for protecting aquatic life and maintaining a healthy ecosystem.
Preventing Acid Rain Pollution
Acid rain forms when pollution from sources like industrial plants and vehicles releases gases such as sulfur dioxide (SO2) and nitrogen oxides (NOx) into the atmosphere. These gases can combine with precipitation to create acid rain. Your proactive efforts in reducing emissions involve using cleaner forms of energy, installing scrubbers on smokestacks, and adhering to pollution control regulations. Transitioning to renewable energy sources such as wind or solar power also plays a pivotal role in tackling the root causes of acid rain. By focusing on these preventative strategies, you contribute to a reduction in pollutants that lead to the formation of acid rain.
Monitoring and Future Outlook
Effective monitoring of rainwater pH levels is critical in gauging the success of policies and practices aimed at mitigating acidic rainfall. You have an integral role to play, whether as a policy maker, industry professional, or concerned citizen, in understanding the impact of acid rain.
National and International Efforts
National efforts, such as those driven by the National Atmospheric Deposition Program (NADP), provide comprehensive monitoring and research on acid rain. Regular data from NADP’s network of sites contributes to a deeper understanding of the trends and geographic distribution of rainwater pH levels. At an international level, there are collaborations across borders that help to share knowledge and tackle challenges more comprehensively, given that air pollution and its effects are not confined by national boundaries.
Industries, cars, and power plants represent primary emission sources that affect acid rain. These sectors are being continuously scrutinized and regulated to limit the release of sulphur dioxide (SO2) and nitrogen oxides (NOx), the precursors to acid rain. Internationally, agreements such as the Gothenburg Protocol under the Convention on Long-range Transboundary Air Pollution work towards reducing these emissions.
Future Challenges in pH Regulation
The future of rainwater pH regulation not only hinges on ongoing emissions control but also on adapting to the new challenges posed by climate change. Studies suggest more winter rainfall is becoming concentrated in intense events, potentially influencing acidity levels. Meeting this challenge requires high-quality, long-term monitoring programs to understand and anticipate changes.
Further, as you move towards cleaner vehicles and renewable energy sources, the role of legacy and natural sources of acidity in the environment becomes more pronounced. To that end, ensuring the transition to green technologies in industries and energy production is crucial to reduce the reliance on fossil fuels and their associated emissions.
Your awareness and actions contribute to shaping the trajectory of these efforts, influencing the health of ecosystems and the quality of the water you rely on.
Frequently Asked Questions
The pH level of rainwater is a crucial factor in understanding environmental chemistry and its impact. These frequently asked questions provide insights into what influences rainwater acidity, its effects, and measurement techniques.
What is Pure Water’s pH?
Pure water is often referred to as having a neutral pH, which is 7. This neutrality comes from water’s equal concentration of hydrogen ions (H+) and hydroxide ions (OH-), which balance each other out. Pure water rarely exists in nature due to dissolved minerals and gases that can change its pH.
What factors contribute to the acidification of rainwater?
Your rainwater’s acidity can be affected by both natural processes, such as volcanic emissions, and human activities such as the combustion of fossil fuels. These sources release sulfur dioxide and nitrogen oxides, which react with water vapor in the atmosphere to form acids.
How does acid rain affect the environment and infrastructure?
Acid rain can lead to the degradation of forests and soils by dissolving and washing away nutrients and minerals. It also corrodes buildings, monuments, and infrastructure, particularly those built with limestone or marble.
What is considered a normal pH range for rainwater?
Normal rainwater pH levels typically range from 5.5 to 6.5. While rain is naturally slightly acidic due to the carbon dioxide in the atmosphere, that forms carbonic acid, values below this range indicate acid rain.
How can one manage and neutralize pH levels in rainwater collection systems?
To manage pH levels in rainwater collection systems, buffering agents like limestone can be used to neutralize acidity. Regular monitoring and maintenance of pH levels ensure that the collected water is safe for intended use.
What is the typical pH level of freshwater sources, and how does it compare to rainwater?
Freshwater sources usually have a pH between 6.5 and 8.5, making most of them more neutral or slightly basic when compared to rainwater. The naturally acidic state of rainwater can alter the pH of freshwater sources, especially in areas prone to acid rain.
What are the methods to measure and monitor the pH of rainwater?
Measuring the pH of rainwater can be done using pH meters, litmus paper, or pH indicator solutions. Monitoring typically involves collecting samples and testing them periodically to track changes and assess the need for pH adjustment.
